|
Seed Germination Studies in Rauvolfia serpentina
U.K. Sinha, M.P. Trivedi and Rachna Kumari
Department of Botany
Patna Science College, Patna University, Patna – 800 005, India
Abstract
Seed germination studies in Rauvolfia serpentina have shown seedcoat dormancy. Unscarified seeds do not germinate at all while scarified seeds exhibit 54% germination. Attempts of scarification by conc. H2SO4 proved futile. There was reduction in germination at higher moisture and salt stress created by NaCl and Na2SO4. In MgSO4, the germination potential was 82.7% at lower concentration as against declining trend at higher concentrations. Unscarified seeds did not germinate at any concentration of IAA as against scarified seeds which showed 95.2% germination at 10ppm and 90.4% at 100ppm. Higher percentage of germination was also achieved in response to Gibberellic acid and Cytokinin at moderate and higher concentrations.
Key Words : Germination, Rauvolfia serpentina
Introduction
Rauvolfia serpentina (L.) Benth.ex kurz (Apocynaceae) is a well known medicinal plant against hypertension as sedative or tranquilizing agent. It is commonly called as Sarpgandha and is widely distributed in sub-Himalayan tract. The plant is an erect evergreen perennating undershrub. Flowers are white with pinkish tube in terminal peduncled bright red cymes. Seeds are polished green in young stages and purplish black at maturity.
Despite their wide geographical distribution and edaphic tolerance, Rauvolfia serpentina is not easily cultivated due to limited supply from wild sources, laborious collection of seeds and poor percentage of seed germination. Sometimes, there is no embryos in fruits of perfectly normal phenotypic appearance. It has been observed that optimum yield of roots (parts used for alkaloids) is obtained when plants are propagated by seeds. Thus, it is essential to study germination potential of this species in varying environmental regimes.
Material and Methods
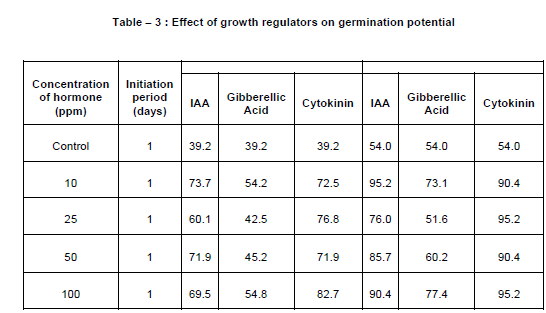
The material for present investigation is Rauvolfia concentration of IAA. Scarified seeds showed 95.2% germination at 10 ppm and 90.4% at 100 ppm. (Table 3)
• Effect of Gibberellic Acid :
Rate of germination varied from 51 to 77.4% at varying concentrations. At 100 ppm the germination was maximum i.e. 77.4%. (Table 3) • Effect of Cytokinin :
At moderate and higher concentrations, the germination potential was 95.2%. The lower concentration (10 ppm) showed 90.4% germination. (Table 3)
Discussion
On perusal of the results, it appears that R. serpentina has an extremely hard seedcoat. Conc. Sulphuric acid treatment failed in making the coat permeable. Seeds having half coat removed by blade proves that dormancy is due to hard seedcoat which prevents the germination of seeds and may be an ecological adaptation (Jain et al, 2006). Seed coat puncturing has been tried in Sida spp. with favourable results (Lissy & Simon, 2007).
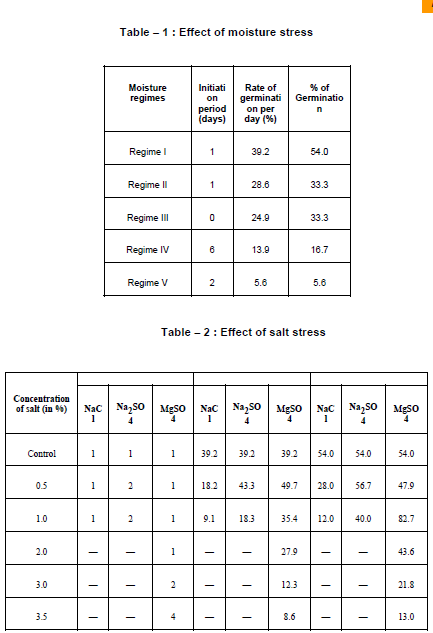
Seeds in response to moisture stress established that availability of water is directly proportional to seed germination. In regime I where water was optimum, the germination potential was 54%. The germination declined in increased moisture stress. Khadeer et al. (1987) explained the arrest of synthetic activity at high stress due to increased activity of oxidase and hydrolysing enzymes. Agarwal et al (1999) have reviewed certain aspects of water stress induced changes and tolerance mechanism in plants. They concluded that solute accumulation, ascorbic acid composition and stimulated functioning photosynthesis are physiologically affected under water stress conditions.
The availability of water for imbibition depends to a large extent on the composition of the medium in which germination takes place. As the concentration of salts in the medium increases, the availability of water for imbibition decreases due to osmotic effects. Kalita and Bhatttacharya (1993) studied effect of phenol on germination and NaCl on germination and seedling growth of pea. They observed reduced length of main root with 4 hrs. soaking period. Almost all concentrations showed reduced length of main root. Length was reduced to 3 cm at 50 ppm concentration. They explained it due to changes of certain metabolic processes. The enhanced germination at low concentration (0.5%) of MgSO4 might be due to utilisation of Mg for synthesis of various storage magnesium salt. Magnesium in plants is mainly related to its capacity to interact with strongly nucleophilic Jain Usha, Rampal Ahrodia and Dinesh Kumar Soni 2006. Seed germination and dormancy breaking techniques for Guazuma tomentosa kunth and Helicteres isora Linn (Family – Sterculiaceae). Indian Journal of Environmental Sciences 10 (1) : 59-61. Joshi, A.J. and Khairatkar, P.P. 1995. Seed germination, amino acids and sugars in seedlings of Juncus maritimus and J. acutus under salt stress. J. Indian bot soc. 74 15-17
Kalita, Mohan Ch. and Bhattacharya, Nizara 1993 Effect of phenol on germination and NaCl on germination and seedling growth of pea (Pisum sativum L.) J.Mendel 10 (2-4), 69-70.
Khadeer, M.A, E. Seshagiri and S.Y. Anwar 1987. Evaluation of the role of ascorbic acid in salt tolerance with different varieties of safflower (Carthamus tinctorius L), Mendel 4 (4) : 225-228.
Kumar Arun, Kaul, B.L. and Verma H.K 2001. Seed germination studies in Withania somnifera (L.) Dunal.
|
|
serpentina. It is source of a drug reserpine used in treating blood pressure and insanity.
The seeds are dormant and suffer from seedcoat dormancy. To study germination, seeds were mechanically scarified with blade and half coats were removed. Care was taken to protect the embryonal portion.
Germination was achieved after treating the seeds with 0.1% HgCl2 and put on moist filter paper backed with cottonwool in Petridishes. Distilled water was used for moistening. The temperature was kept at 35ºC. In moisture stress experiments, artificial stress was created by increasing the number of filter papers keeping the amount of water constant (Sinha et al., 1991). IAA, GA3 and Cytokinins were used in 10, 25, 50 and 100 ppm concentrations.
Observation
In control, the germination potential is zero in unscarified as against 54% in scarified seeds. • Effect of scarification by conc. H2SO4 :
Seeds were scarified with conc. H2SO4 for 2 minutes to 20 minutes. They were washed thoroughly in running water for an hour to remove traces of H2SO4. Seeds thus, washed were kept for germination. Seeds treated with conc. H2SO4 did not germinate at all. • Effect of Moisture Stress : Seeds showed 54% germination in regime I which acted as control. There was 33.3% germination in regimes II and III. (Table 1) • Effect of Salt Stress : At lower concentration of NaCl i.e. at 0.5%, the seeds showed 28% germination but at moderate concentration, the germination decreased. At 2-3.5%, the seeds did not germinate. (Table 2) In Na2SO4, the germination percentage is 28-56.7% at 0.5% concentration. The rate of germination decreased and became nil at higher concentrations. In MgSO4, the germination potential was 82.7% at lower concentration as against declining trend at higher concentrations. • Effect of IAA : Unscarified seeds did not germinate at any ligands through ionic bonding and to act as a bridging element or form complexes of different stabilities (Marschner, 1995). It may be safety concluded that soils having low sulphate are suitable for better germination potential of Rauvolfia serpentina. Joshi and Khairatkar 1995 studied seed germination, amino acids and sugars in seedlings of Juncus spp under salt stress. They reported adverse accumulation of total and reducing sugars and that of glucose, galactose and arabinose in both the species. There was greater concentrations of asparagines, aspartic acid, phenylalanine and of some other aminoacids including proline in seedlings in saline habitat. These amino acids have protective effects on cell membranes in seedling in halophytes.
Germination is greatly influenced by endogenous and exogenous growth hormones. Unscarified seeds did not germinate at any concentrations of IAA while scarified seeds germinated upto 95.2% at 10 ppm of IAA and moderate and higher concentrations of cytokinin. At 100 ppm of IAA, the germination was 90.4%. Lower concentration of cytokinin was also promotive. In GA3 treatment, the germination varied from 51 to 77.4%. Effect of different physical and hormonal treatments on the release of dormancy shows that compared to physical treatment, hormonal treatments were more effective in Withania somnifera, (L.) Dunal by Kumar et al (2001).
It appears that plant hormones affect nucleic acid directed protein synthesis, enzyme activity and membrane permeability either directly or through some other metabolites. The molecular aspects include activation of gene expression and sticking to the lipid components of the membranes and altering the membrane permeability (Srivastava, 2005). The promotive but lesser percentage of germination in Rauvolfia serpentina after GA3 treatment is due to keeping the seeds at 35ºC for germination. According to Srivastava (2005), GA3 promotes seed germination in several spp which otherwise fail to germinate unless subjected to low temperature, long days or red light. Overall seed germination in Rauvolfia serpentina is low. Very poor germination of seeds on filter paper may be ascribed, apart from other factors including deficient food reserves, primarily to the seedcoat factor.
References
Agarwal, R.M, Pandey Rashmi and Gupta Sunita 1999. Certain aspects of water stress induced changes and tolerance mechanisms in plants. J. Indian bot soc 78 255-269. Int. J. Mendel 18 (4) 111-112. Lissy K.P and Thara K. Simon 2007. Viability, germination and reproductive capacity of different species of Sida Linn and its substitutes. Eco. Env. & Cons. 13 (4) : 843-846.
Marschner, W. 1995. Functions of Mineral Nutrients : Macronutrients. In Mineral nutrition of Higher plants (2nd edn.). Academic Press, London pp 277-284. Sinha, R.P, A.K. Mishra and M.P. Trivedi 1991. Seed dormancy and germination in Melilotus spp. Recent Researches in Ecology, Environment and pollution (Eds R.N. Trivedi, P.K. Sen Sarma and M.P. Singh) Today & Tomorrow's Printers and Publishers, New Delhi, 5 : 203-214.
Srivastava, H.S. (2005) Plant Physiology, Rastogi Publications, Meerut.
|
|



