Introduction
Mass spectrometry is an analytical technique for measuring the mass of chemical (and atomic) species. Mass spectrometry has been described as the smallest scale in the world, not because of the mass spectrometer’s size but because of the size of what it weighs. A mass spectrometer determines the mass of a molecule by measuring the mass-to-charge ratio (m/z) of its ion. Ions are generated by inducing either the loss or gain of a charge from a neutral species. Once formed, ions are electrostatically directed into a mass analyzer where they are separated according to m/z and finally detected. The result of molecular ionization, ion separation, and ion detection is a spectrum that can provide molecular mass and even structural information. Mass spectrometers constitute a large, very diverse, and widely employed and produced class or family of instruments. It is likely that no other type of complex instrument has been as important for so many fields of science in the twentieth century.
History of Mass Spectrometry
Today's mass spectrometer is based on the seminal work performed by Sir J. J. Thomson of the Cavendish Laboratory of the University of Cambridge. Thomson's research, which led to the discovery of the electron in 1897, also led to the first mass spectrometer while he was measuring the effects of electric and magnetic fields on ions generated by residual gases in cathode ray tubes. Thomson noticed that the ions move through parabolic trajectories proportional to their "mass-to-charge" ratios. Thomson received the 1906 Nobel Prize in Physics "in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gasses."
The time period from the late 1930's to the early 1970's was a time of great achievement in the field of mass spectrometry. By the end of World War I, the work of Francis W. Aston and Arthur J. Dempster brought improved higher accuracy mass spectrometers into reality. Later, Alfred Neir incorporated these developments
Mass spectrometers can be divided into three fundamental parts, namely the ionisation source, the analyser, and the detector. 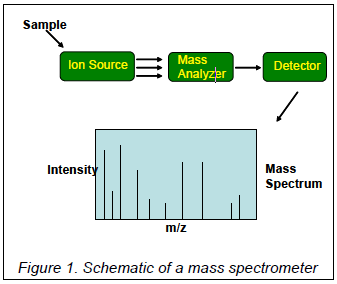
The sample under investigation has to be introduced into the ionisation source of the instrument. Once inside the ionisation source the sample molecules are ionised, because ions are easier to manipulate than neutral molecules. These ions are extracted into the analyser region of the mass spectrometer where they are separated according to their mass (m) -to-charge (z) ratios (m/z). The separated ions are detected and this signal sent to a data system where the m/z ratios are stored together with their relative abundance for presentation in the format of a m/z spectrum.
The analyser and detector of the mass spectrometer, and often the ionisation source too, are maintained under high vacuum to give the ions a reasonable chance of travelling from one end of the instrument to the other without any hindrance from air molecules. The entire operation of the mass spectrometer, and often the sample introduction process also, is under complete data system control on modern mass spectrometers.
Methods of Ionization
Ionization is a process of producing an electrically charged protein molecule from a neutral protein molecular by either adding protons or removing electrons. The different ionization methods, listed below, work by either ionizing a neutral molecule through electron ejection, electron capture, protonation, cationization, or In this method, the sample to be analyzed is mixed with a matrix compund and crystallized. This matrix compound is usually a weak inorganic acid. This mixture is then excited with a laser which results in the evaporation of the matrix compund. The matrix compound also carries the sample molecules into the vapour phase, resulting in indirect vaporization of the sample. Sample ions are formed by the exchange of electrons and protons with the matrix compound. MALDI is especially useful for protein and peptide identification using masses alone since the masses of ions can be determined with great accuracy.

Application of Mass Spectrometry
Mass spectrometers are used in industry and academia for both routine and research purposes. The following list is just a brief summary of the major mass spectrometric applications:
•Biotechnology: the analysis of proteins, peptides, oligonucleotides •Pharmaceutical: drug discovery, combinatorial chemistry, pharmacokinetics, drug metabolism •Clinical: neonatal screening, haemoglobin analysis, drug testing •Environmental: PAHs, PCBs, water quality, food contamination •Geological: oil composition
Mass spectrometers have become pivotal for a wide range of applications in the analysis of inorganic, organic, and bio-organic chemicals. It is being continually improved and has recently had significant advances in its application to molecular biology, where it is now possible to perform |
|
along with advances in vacuum technology and electronics to greatly decrease the size of mass spectrometers. In 1946, William E. Stephens proposed the concept of time-of-flight analyzers, which also separated ions by measuring velocities as the ions move in a straight path toward a collector. The other analyzer in use today, the quadrupole analyzer, was developed in the mid-1950s by Wolfgang Paul. This analyzer is capable of separating ions with an oscillating electrical field further increasing the utility of mass spectrometers. Another Paul innovation was the quadrupole ion trap, which is a device specifically designed to trap and measure ions. The first ion trap became available commercially in 1983 and now both the quadrupole and quadrupole ion trap are the most widely used mass analyzers in the world and for his innovative work Paul was awarded the 1989 Nobel Prize in Physics. This era of rapid development fueled the enthusiasm that ushered in even greater discoveries during the 1980s and 1990s.
Two techniques developed in the mid 1980's, electrospray ionization (ESI) and matrix assisted laser desorption/ionization (MALDI), have had a significant impact on the capabilities of mass spectrometry. ESI is the production of highly charged droplets that are treated with dry gas or heat to facilitate evaporation, which eventually eject the ions in the gas phase. ESI was first conceived by Malcolm Dole in the 1960's yet, by incorporating technology that had become available over the years, John Fenn put ESI into use for biomolecule analysis in the 1980's. MALDI was developed by Tanaka et al. (Japan) as well as by Franz Hillenkamp and Michael Karas, both of Germany. In 2002, the Nobel Prize in Chemistry was received by John Fenn for the development of electrospray ionization (ESI) and Koichi Tanaka for the development of soft laser desorption (SLD) in 1987. MALDI uses a laser to desorb sample molecules from a solid or liquid matrix containing a highly UV-absorbing substance.
Basic Components of a Mass Spectrometer deprotonation, or by transferring a charged molecule from a condensed phase to the gas phase. Many ionisation methods are available and each has its own advantages and disadvantages. The ionisation method to be used should depend on the type of sample under investigation and the mass spectrometer available.
Atmospheric Pressure Chemical Ionisation (APCI)
Chemical Ionisation (CI)
Electron Impact (EI)
Electrospray Ionisation (ESI)
Fast Atom Bombardment (FAB)
Field Desorption / Field Ionisation (FD/FI)
Matrix Assisted Laser Desorption Ionisation (MALDI)
Thermospray Ionisation (TSP)
Electrospray Ionisation (ESI) and Matrix Assisted Laser Desorption Ionisation (MALDI)
The ionisation methods used for the majority of biochemical analyses are ESI and MALDI. ESI is a newer method of ionization that does not cause excessive fragmentation. ESI generates ions directly from solution without requiring any heating. Therefore, this method can be used for heat-sensitive molecules which cannot be ionized by heating. Sample is sprayed in a fine spray in the presence of an electric field. Charge accumulates on the sample droplets which then explode due to mutual repulsion of charges leading to the formation of ions. Both singly and multiply charged ions can be produced in this manner.
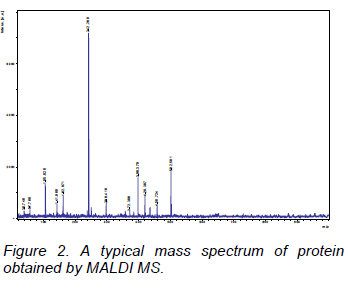
MALDI is another method of ionization that does not require any heating for the production of ions and can be used for heat sensitive molecules
sequencing of peptides and proteins; studies of noncovalent complexes and immunological molecules; DNA sequencing; and the analysis of intact viruses.
ESI and MALDI mass spectrometry have allowed for sophisticated applications of mass spectrometry to the fields of biology and medicine. ESI allows for very sensitive analysis of small, large and labile molecules such as peptides, proteins, organometallics, oligosaccharides, and polymers. ESI has made liquid chromatography mass spectrometry routine, adding a new dimension to the capabilities of liquid chromatography characterization. MALDI has had its wide impact on the field of protein research. The ability to generate MALDI-MS data on whole proteins and proteolytic fragments is extremely useful for protein identification and characterization. The trend toward mass spectrometry as the technique of choice for identifying and probing the covalent structure of proteins is accelerated by the genome project. Genomics demonstrated the power of high-throughput, comprehensive analyses of biological systems. Genomics also provides complete genomic sequences, which are a critical resource for identifying proteins quickly and robustly by the correlation of mass spectrometric measurements of peptides with sequence databases. As the human genome is completed, it can be argued that the characterization of genomic proteins (a field of research also known as "proteomics") is the most important application of modern mass spectrometry.
About the Author: Mohammad Abul Farah attended Aligarh Muslim University in India where he received his M.Sc. and Ph.D. in Zoology with specialization in Genetics. He also served as Senior Research Fellow of Council of Scientific and Industrial Research, India. At present, he is working as a Research Scientist in Proteonik Inc., a biotechnology venture company based in Seoul, South Korea, on Diabetes research focusing on insulin signaling pathway. He can be reached at:farahkorea@yahoo.com |