1.1 General description on solar cell technology
The top ten problems mankind will face during the next fifty years according to Nobel laureate Richard smalley of Rice University, Houston, USA are
1. Energy 2. Water 3. Food 4. Environment 5. Poverty 6.Terrorism 7.Di sease 8. Education 9. Democracy 10. Population
1. Energy 2. Water 3. Food 4. Environment 5. Poverty 6.Terrorism 7.Di sease 8. Education 9. Democracy 10. Population The quality of human life largely depends on the availability of energy sources. The present annual worldwide energy consumption has already attained a level of over 400 exajoules and is expected to further augment steeply from the increase in world population and the rising demand of energy in the developing countries. So solving energy need would be the top priority. The quality of human life largely depends on the availability of energy sources. The present annual worldwide energy consumption has already attained a level of over 400 exajoules and is expected to further augment steeply from the increase in world population and the rising demand of energy in the developing countries. So solving energy need would be the top priority. Currently we derive energy mainly from coal, gas and oil, which are non-renewable energy sources. According to estimated sources the reserves of these fossil fuels will deplete during this century. There has been a worldwide scientific movement to develop a global society, which employs sustainable methods in all aspects of life. One of these methods is to use renewable energy. Sun, Wind and Wa ter are the renewable energy sources. Fortunately, the supply of energy from the sun to the earth is gigantic; 3 × 10 24 joules/year or about ten thousands times more than what mankind consumes currently. To tap into this huge energy reservoir of the sun remains, nevertheless, a major challenge for mankind. Currently we derive energy mainly from coal, gas and oil, which are non-renewable energy sources. According to estimated sources the reserves of these fossil fuels will deplete during this century. There has been a worldwide scientific movement to develop a global society, which employs sustainable methods in all aspects of life. One of these methods is to use renewable energy. Sun, Wind and Wa ter are the renewable energy sources. Fortunately, the supply of energy from the sun to the earth is gigantic; 3 × 10 A structure that conver ts directly sunlight (solar energy) into usable electric energy is called a solar cell. They are also referred as photovoltaic (PV) cells where PV stands for Photo (light) and Voltaic (electricity). They are useful because they are silent; have no moving parts; cause no environmental pollution in operation; never run out; can generate power where it is needed , without the need for electricity pylons and wires; work in cloudy weather and are good for low temperature climate. Use of solar cell may help in reduction or prevention of the use of fossil fuels (Oil, gas, wood, coal) and thereby further prevents emissions of CO2 (glasshouse effect), SO2, NOx (acid rain), etc. by conv entional power stations. A structure that conver ts directly sunlight (solar energy) into usable electric energy is called a solar cell. They are also referred as photovoltaic (PV) cells where PV stands for Photo (light) and Voltaic (electricity). They are useful because they are silent; have no moving parts; cause no environmental pollution in operation; never run out; can generate power where it is needed , without the need for electricity pylons and wires; work in cloudy weather and are good for low temperature climate. Use of solar cell may help in reduction or prevention of the use of fossil fuels (Oil, gas, wood, coal) and thereby further prev PV-powered dollar bill changers and devices that decoded computer punch cards and tape. In 1958, first PV powered satellite Vangaurd-I was launched. First Gallium Arsenide (GaAs) solar cell produced. Since then ther e is no looking back in harnessing the vast solar energy for mankind. D. E. Carlson (1976) and Jerry Olson (1987) discovered cheap and efficient mode of converting sunlight into electricit y by utilizing amorphous silicon and fabricating multi-junction, respectively with an efficiency of 20 and 34%. These modern solar cells are basically p-n junction photodiodes, made of basically silicon for most of the industrial purposes. Emergence of a new class of semiconductors (also known as highly mis- matched alloys, e.g. Zn 1-y Mn y O x Te 1.x ) and the recent efforts made by scientists at Berkeley and at MIT using them has develop a new class of solar cell with power conversion efficiency of 56%. The method used to synthesize these semiconductors for solar cell purposes (Yu, K. M. et al. Phys. Rev. Lett. 2003 , 91 , 246403) is very tedious and is highly expensive. 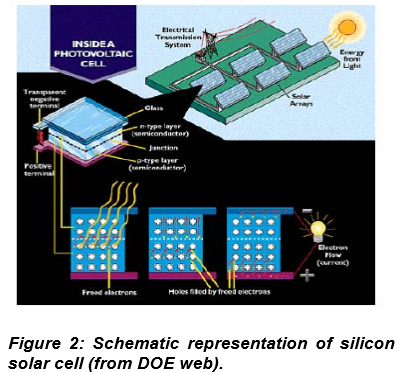
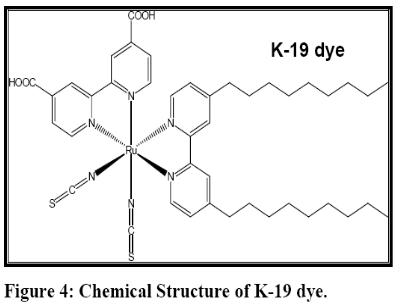
To give the moving electrons the clearest path to the electrode, the semiconductors in the best conventional solar cells must be highly pure and defect free – thus expensive to produce. Dye sensitized solar cells adopt a low cost solution to the problem of purity by separating the two jobs of collecting light and ferrying electrons. The overall power conversion efficiency of nearly 10-12% has been achieved by varying the film morphologies, incorporating hydrophobic tails into light sensitive substance, K-19 (Figure 4) and using solvent free electrolyte.
The critical stability test of 1000 hours at 80 ºC with this cell showed 6% of conversion efficiency losing only 4% of its performance. Unlike amorphous silicon, a recent stability test of 12,000 hours with this cell upon full-intensity light exposure showed no sign of photo degradation.
2.2 Dye-Sensitized solar cell: Components DSSCs are extremely promising because they are made of low-cost materials and do not need elaborate apparatus to manufacture. Several scientific groups have giv en lots of attention and contribution on developm ent of each component. Figure 3 is the schematic representation of DSSC having four major components. They are
1. Transparent ITO coated glass
2. Granular TiO
forming a nonporous structure.
3. A dye (N719), which is a light-sensitive substance spread on the TiO
2 surface.
4. A redox couple located in the space between the dye and the cathode.
pathways in the DSSCs. In this section basic processes that occur in the photo induced interfacial electron transfer from the K-19 dye to the TiO 2 semiconductor in solution (no redox couple) are described. Although the processes in this specific case are for K-19-TiO 2 film, to some extent the processes can also be generalized to other sensitizer-sem iconductor systems. Processes under illu mination are:
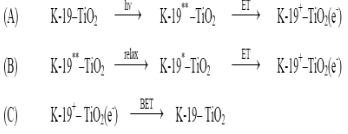 The K-19 dye captures a photon (h ? ),which initiates a long-lived charge separated state when an electron from the electr onically excited state of the dye is injected into the conduction band of the semiconductor (Equations A and B). The two different electron transfer pathways indicate the possibility of electron in jection from the fully relaxed excited state (K-19 * ), as well as from non- thermalized, higher lyi ng excited states (K-19 ** ). When an electron is injected, the dye cation (K- 19 + ) is formed together with an electron in the conduction band of the titanium dioxide (TiO 2 (e - )). Back-electron transfer (BET) from the conduction band/trap states of the semiconductor to the dye cation restores the original ground state of the dye (Equation C). The forward electron injection process (Equations A and B) occurs on femtosecond and picoseconds time scales while the BET occurs non-exponentially on the microsecond to millisecond time scale with only a negligible contribution from picoseconds/nanosecond components. The difference of several orders of magnitude between the time constants of electron injection and BET is one of the important properties which make the dye-TiO 2 system one of the most efficient light-to- energy converters available for DSSC. The K-19 dye captures a photon (h ? ),which initiates a long-lived charge separated state when an electron from the electr onically excited state of the dye is injected into the conduction band of the semiconductor (Equations A and B). The two different electron transfer pathways indicate the possibility of electron in jection from the fully relaxed excited state (K-19 * ), as well as from non- thermalized, higher lyi ng excited states (K-19 ** ). When an electron is injected, the dye cation (K- 19 + ) is formed together with an electron in the conduction band of the titanium dioxide (TiO 2 (e - )). Back-electron transfer (BET) from the conduction band/trap states of the semiconductor to the dye cation restores the original ground state of the dye (Equation C). The forward electron injection process (Equations A and B) occurs on femtosecond and picoseconds time scales while the BET occurs non-exponentially on the microsecond to millisecond time scale with only a negligible contribution from picoseconds/nanosecond components. The difference of several orders of magnitude between the time constants of electron injection and BET is one of the important properties which make the dye-TiO 2 system one of the most efficient light-to- energy converters available for DSSC.
A key issue in photo induced processes of dye-sensitized semiconductor films has been the mechanism of the ultra fast, sub-hundred femtosecond time scale electron injection, especially, whether it occurs from non-thermalized states prior to energy equilibration over all |
|
emissions of CO2 (glasshouse effect), SO2, NOx (acid rain), etc. by conv entional power stations. 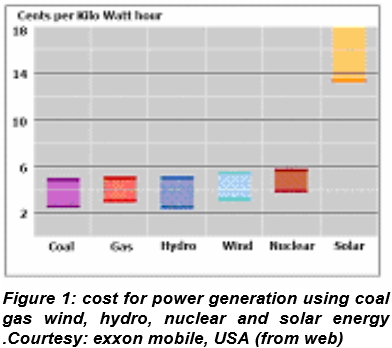 1.2 Background of Invention 1.2 Background of Invention
The development of the solar cell technology begins with the 1839 research of French experimental physicist Antoine-Cesar Becquerel. He discovered (although he could not fully explain) the photovoltaic effect while experimenting with an electrolytic cell containing two metal electrodes. The Then “nineteen year old physicist” found that cert ain metals and solutions would produce small amounts of electric current when exposed to light. According to Encyclopedia Britannica the first genuine solar cell was built around 1883 by Charles Fritts, who used junctions formed by coating selenium (a semiconductor) with an extremely thin layer of gold. Fritts's devices were very inefficient, transforming less than 1 percent of the absorbe d light into electrical energy, but that was a start. Solar cell efficiency finally saw substantial progress with the invention of the first silicon cell by Russell Ohl in 1941.The era of modern solar cell technology began since 1954, when three American researchers, G. L. Pearson, D. Shapin and C. Fuller (Bell Labs.) demonstrated first refined solar cell capable of a 6% energy conversion efficiency with direct sunlight. Western Electric brought PV technology in the market; early successful products included integrated Photovoltaic, Roof-mounted Photovoltaic Standalone P hotovoltaic systems, etc. for PV module preparation, a great source of solving energy.
2.1 Dye-Sensitized solar cell: General
Dye-sensitized solar cell (DSSC) has opened a new corridor, departing, in principle, from classical solid state junction devices and is now challenging traditional and dominant silicon based solar cell industry. DSSCs are photo electrochemical cells that use photo-sensitization of wide-band-gap mesoporous oxide semiconductors. These cells were invented by Michael Graetzel et al. in 1991 and are also known as Grätzel cells. [B.O. Regan and M. Grätzel. A low-cost high-efficiency solar cell based on dye-sensitized colloidal TiO 2 thin film, Nature 353 , 737-40 (1991)], Figure 3.
Solar power visionaries have long dreamed of solar panels that are cheap, efficient and able to withstand the relentless heat of the midsummer sun. Dye Sensitized Solar Cells uses cheaper starting materials than the traditional silicon solar cells to generate power at moderate efficiency of 12%. In conventional solar cells, a semi-conductor material - usually crys talline silicon - absorbs photons of light. The photons give electrons in the material an energy kick that makes them flow through the semiconductor to an electrode, where they can be connected to an electronic circuit and used to perform work. As the electrons race through the semiconducto r, however, they risk running into “holes” left by other electrons. When that happens, electrons and holes can easily recombine, causing the electrons to shed their extra energy as heat 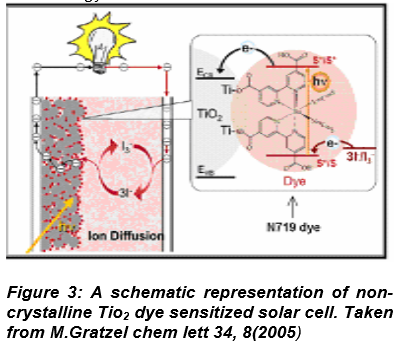
5. A solvent for the redox couples (I - /I 3 - ) e.g. an organic solvent or Room Temp. Ionic Liquid. 6. Pt, cathode
The cells have been compared to photosynthesis because they use the redox reaction of the electrolyte. The energy conversion efficiency of the cells has not yet reached the level of silicon solar cells . The current energy conversion efficiency is about 12%, as was reported by Graetzel et al. It is said that the energy conversion efficiency can rise to 33% in theory.
2.3 Dye-Sensitized solar cell: Operation
Upon passing the solar light through an electrically transparent conductive glass electrode, photo excitation of dye molecules adsorbed onto the surface of sintered nanocrystalline TiO 2 takes place. The excited electron makes a jump from the dye to the conduction band in TiO 2 . This jump occurs very rapidly; it takes only 10 -15 seconds. In TiO 2 , the electron percolates through the TiO 2 film, reaches the glass electrode, goes through external circuit, and reaches the counter electrode. At the same time dye regeneration takes place by receiving one electron from an iodide ion, in turn oxidizing the iodide to triodide. Iodide ion regenerates simultaneously upon receiving one electron from counter electrode, thereby completing the circuit.
2.4 Dye-sensitized solar cell-Electron transfer processes
The most important component of the DSSC is the photoactive electr ode, the dye-sensitized nanocrystalline semiconductor film on a transparent conductive substrate, where the primary step of solar energy into electricity conversion takes place, i.e. electron injection from the photo-excited dye(K-19 ) to the semiconductor, a process that separates the electron and hole. For good photovoltaic performance of the DSSC it is important that absorption of the photon and the ensuing electron injection into the conduction band of the semiconductor (Processes 1 and 2 in Figure 5) occur several or ders of magnitude faster than recombination of the injected electrons with the oxidized dye or the redox system (Processes 6 and 7 in Figure 5). Also, charge transfer between the oxidized dye and the electrolyte (Process 4 in Figure 5) should be much faster than recombination reactions to minimize wasteful electronic, vibrational, and rotational degrees of freedom. Our research has provided evidence for non-thermalized electron transfer from dye to conduction band of the semiconductor.
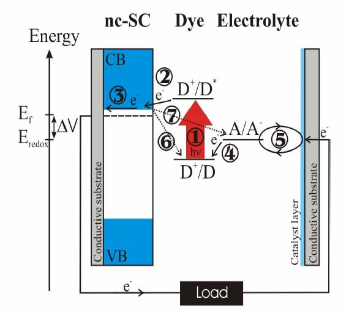
Figure 5: A scheme of the operation of the DSSC, solid arrows represent the primary and dashed arrows the wasting pathways in the solar cell. Following the excitation of the dye (process 1), electrons is injected into the conduction band of the non crystalline semiconductor [nc-sc] (process 2) where they can move through the network of nano particles (process -3). The oxidized dye molecule receives an electron from the mediator, a radox species in the electrolyte (process 4) and oxidized electrolyte species diffuse to the cathode where they are reduced by the electrons circulated through the external circuit (Process 5). The recombination of injected electrons is sown as the dashed arrows (process 6 and 7).
This is how dye-sensitiz ed solar cell receives solar energy and gives electric current to an external circuit.
About the Author: Dr. Sudhir Ranjan is working as scientist at KMG2 Sensors Corp., State College, Pennsylvania, USA. Email: ransud@yahoo.com |