Surface analysis of steel samples polarized anodically in H3PO4 -HCl mixtures by SEM and EDAX techniquesSurface analysis of steel samples polarized anodically in H3PO4 Rita Khare Department of Chemistry Government women's College Gardanibagh, Patna, Bihar, India Abstract Potentiodynamic polarization experiments were performed on 304 stainless steel in 14M H3PO4 containing Key words: Stainless steel; pitting; Scanning Electron Microscopy; Energy Dispersive Introduction Present study involves surface analysis of AISI 304SS after treatment in H 3PO4 - HCl medium. It is observed that stainless steels which are quite resistant to corrosion in various mediums [1] and also in presence of H3PO4 (due to formation of passive film ), suffer severe destruction in the form of pitting if the medium has aggressive ions like The corrosion behaviour of 304SS in concentrated phosphoric acid having different concentrations of added impurity in the form of HCl at different temperatures has been described earlier [3,4]. It was inferred from these studies that at a certain temperature, there is a certain critical concentration of HCl below which pitting doesn't occur but at and above critical concentration of HCl localized attack on alloy surface in the form of pitting is witnessed. Hence the presence of aggressive ion in the form of HCl limits the utilization of 304SS in this medium. Surface analysis of steels has been done earlier by investigators but Scanning Electron photomicrographs of steels having other compositions are available in literature [5,6]. Furthermore earlier studies were carried out primarily to study stress corrosion, cracking and pitting in other mediums. Hence it seems desirable to study the severity of pitting and effect of temperature on it by surface analysis of steel samples after exposing them to Materials and Methods Test samples were prepared from stainless steel sheets obtained from M/s Goodfellow Metals Ltd, England. The composition of 304SS was 18 Cr, 10 Ni and balance Fe. Test samples were polished and cleaned as mentioned elsewhere [3,4]. The electrode system consisted of the austenitic stainless steel working electrode, a counter electrode of platinum and a saturated calomel electrode (SCE) with KNO3 salt bridge. The electrochemical experiments were carried out in an air thermostat maintained at 298, 308 and 318 0 K under still condition. The solution consisted of 14M phosphoric acid with different ppm of HCl (BDH AR Grade) added to it. Potentials were impressed on the working electrode by a fast power potentioscan Wenking model POS 73. Potentiodynamic polarization experiments were recorded at scan rate of out after the anodic polarization experiments. For this Scanning Electron Microscope Energy dispersive
entered a wafer of pure silicon carefully treated with lithium. As the Results and Discussion The Potentiodynamic polarization curves obtained by anodic polarization of 304SS at different temperatures have been shown elsewhere [3]. It is observed that irrespective of concentration of HCl and temperature, nature of the potentiodynamic polarization curves is same. It has an active region followed by a passive range and then the curves enter into transpassive region indicating dissolution of passive film. When the concentration of HCl is less than certain critical value steel surface doesn't undergo pitting attack but at and above critical concentration of HCl pitting corrosion takes place (it is ascertained by SEM analysis of alloy surface after the experiment). The critical concentration of HCl at which pitting starts is found to be 7600 ppm at 2980K, 3800 ppm at 308 0K and 2900 ppm at 318 0K [3,4]. It is observed that at a lower temperature a higher concentration of HCl may be tolerated by alloy without suffering destructive attack in the form of pitting. It has been discussed earlier that the resistance against pitting offered by Mo containing stainless steel alloy in this medium also depends on temperature in a similar manner [7,8]. The photomicrographs of test samples obtained from Scanning Electron Microscopy after performing polarization experiments on 304SS in 14M phosphoric acid and in phosphoric acid containing 7600 ppm HCl at 298 0K are shown in fig Table 1 Atomic percentage of different elements in 304SS obtained from EDAX. 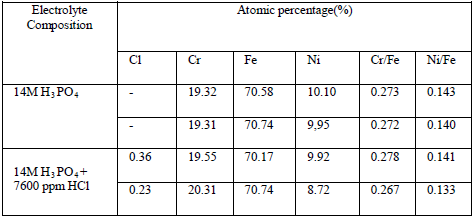
When Cl- ions are present in the medium, a very small amount of corrosion takes place in spite of the passive state of the alloy [9]. As the formation of metal oxide film progresses, anodic areas are reduced to very small dimensions. The film growth is due to interaction among metal cations and oxygen ions. The rate of transport of cation vacancies and anion vacancies decide the growth of passive film. Anion vacancies are generated at metal film interface and consumed at film solution interface. At a higher temperature, rate of generation of anion vacancies is more but rate of their consumption by oxygen ions slows down. This is due to the presence of aggressive chloride ions in the medium, which adsorb competitively at anion vacant sites. This in turn creates cation vacancies [10]. At a higher temperature, cation vacancies are generated rapidly due to stronger chemisorptions of halide ions at film solution interface. Due to presence of more cation vacancies and anion vacancies the formation of compact passive film is restricted. This leads to the formation of more porous passive film at a higher temperature as anodic areas are more and density of vacancies is greater [7]. At more noble potentials when metal holes start piling up, formation of void takes place at metal film interface and pitting starts when void grows beyond a certain size. At a higher temperature, pitting starts at several local sites resulting in more number of pits. Size of pits is smaller at a higher temperature as adsorption of halide ions on film surface is limited. Spectra of 304SS obtained by EDAX analysis after treatment in 14M H3PO4 and in 14M 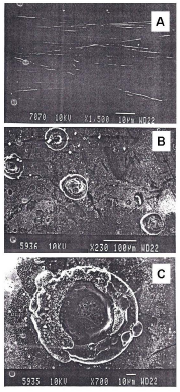
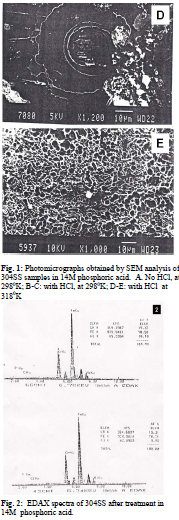
surface of steel samples besides Fe, Cr and Ni. From EDAX analysis of steel samples it is found that 0.23% - 0.36% chlorine atoms are present in passive film Fig. 1: Photomicrographs obtained by SEM analysis of 304SS samples in 14M phosphoric acid. A. No HCl, at 2980K; Fig. 2: EDAX spectra of 304SS after treatment in 14M phosphoric acid. 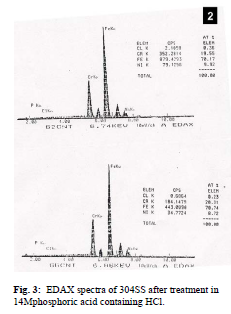
Fig. 3: EDAX spectra of 304SS after treatment in 14Mphosphoric acid containing HCl. Conclusions It is found from SEM analysis that when concentration of HCl is below critical concentration, pitting doesn't take place. At and above critical concentration pitting corrosion is evidenced. The morphology of pits formed remains the same in all the cases i.e. they are partially hemispherical. At higher temperature more pits having smaller size are found to be present. Porosity of passive film depends on the temperature being more porous at a higher temperature. It is inferred from EDAX analysis that when steel samples are treated in HCl containing medium, chlorine atoms are present in the passive film. This supports the view that oxide ion vacancies are occupied by chloride ions up to some extent from the very beginning and continues so long as the passive film formation is completed. The defect density in the passive film leads to the formation of pits ultimately. Acknowledgements Author gratefully acknowledges the experimental facilities provided by Institute of Technology B.H.U., Varanasi. Facilities for SEM and EDAX provided by Dept of Metallurgy, I.T., B.H.U. and Department of Physics, B.H.U. respectively are also gratefully acknowledged. Discussions with Dr M.M. Singh and Dr A.K. Mukherjee, from I.T., B.H.U. were fruitful in the preparation of this paper. References 1.A. Alon, J .Yahalom and M. Schorr, Corrosion, 31, 315 (1975). 2.M.M. Badran, Corrosion prev and control, August 97 (1987). 3.M.M. Singh, A.K. Mukherjee and Rita Khare, Bulletin of Electrochemistry, 11 (10) 457 (1995). 4.Rita Khare, A.K. Mukherjee and M.M. Singh, SAEST, 29, 118 (1994). 5.E.A. Abd El Meguid , A.A. Abd El Latif, Corros. Sci., 46, 6.E.A. Abd El Meguid, N.A. Mahmoud and V.K. Gouda, British. Corros. J, 33 (1) 7.J.H. Wang, C.C Su and Z. Szklarska. Smialowska, Corros. Sci., 44, 10 (1988). 8.M.M Singh, Rita Khare and A.K. Mukherjee, Indian Journal of Chemical Technol., 9, 407 (2002). 9.Rita Khare, Ideal Research Review, 27, Sept, 21 (2010). 10.I.F. Lin, C.Y. Chao and D.D. Macdonald, J. Electrochem. Soc., 128, 1195 (1981). Corresponding Author: Email: ritre@rediffmail.com |
|||||||

