Biodeterioration of active principles of Catharanthus roseus by Colletotrichum gloeosporoidesS. K. Pandey and A. K. Roy University Department of Botany T.M. Bhagalpur University, Bhagalpur, Bihar, India Abstract Majority of the members of Coelomycetes are diseases causing mostly associated with leaf spots. Leaf spot diseases are the expressions of abnormal metabolic changes due to loss of photosynthetic area. Such symptoms are produced as a result of physiological disorders in the host. These disorders may be due to nutritional imbalance or due to living organisms, usually active in localized areas of the host. Leaf spot diseases are known to cause severe damage to the plants of great economic and ornamental value. Cultivated plants are usually more susceptible to such diseases. These diseases, which deprive the plants of the aesthetic value and cause a decrease in photosynthetic area, require greater probe and attention. A general survey undertaken by the research team of TMBU under AICOPTAX Program, MoEF, New Delhi showed greater frequency of Coelomycetous fungi. Particularly Colletotrichum gloeosporoides which have been selected in the present research work for the study in detail. A general pattern of decrease of sugars, biomass and alkaloid contents were observed after pathological studies of C. roseus. The percentage decrease in Total Sugar was found 30.36%, Reducing Sugar 28.57%, Key words: Coelomycetes, Biodeterioration, Medicinal plants. Introduction The Coelomycetes are predominantly leaf spotting fungi though many of them grow on twigs, fruits and other parts of the plants causing different symptoms like blight, rot, cankers etc. The pycnidia are with or without the ostiole and the acervuli are saucer shaped structure which are the characteristics of this group. The present work deals with taxonomical and pathological studies of Coelomycetous fungi associated with medicinal plants. The main object of the present investigation was to explore the diverse localities of Eastern Bihar to collect different nature of genera of Coelomycetous fungi associated with medicinal and the plants of other economic importance. As early as in 1972 Vidyashekharan and Kandaswamy [1] showed severe reduction in both starch and sugar contents of infected tissue of Phaseolus aureus. The observation [2] supported this findings the facultatine parasite such as Cercospora was found to deplete starch and sugar contents in groundnut and banana leaves [3], also found a quick decline in sugar content of banana due to Cercospora infection. The simple sugars are preferably assimilated by pathogens as carbon source which determines the quality of the fruits and leaves Materials and Methods Estimation of Total Sugar: To 200 mg of each sample, 25 ml hot 80% ethanol was added and stirred thoroughly. After 5 minutes, it was centrifuged and supernatant was decanted. Extraction was repeated by adding 30ml of hot 80% ethanol. The extracts were mixed and ethanol was evaporated to dryness in an evaporating disc. The residue left at the bottom was dissolved in 5ml of Estimation of Reducing Sugar: 300mg of each sample was finely crushed and blended with 1.5ml of glass distilled water in a glass homogeniser. To this 0.2ml of 0.3N Barium hydroxide solution was added followed by 0.2ml of 5% ZnSO4 solution and was thoroughly mixed. The total volume was centrifuged. To one ml of the supernatant, one ml of alkaline copper reagent (Prepared by dissolving 4 gm CuSO4, 5H2O; 24 gm Anhydrous Na2CO3 and 16 gm Estimation of Loss in Biomass: The test plant (Catharanthus roseus) was inoculated artificially by the test fungus (Colletotrichum gloeosporoides). The plant after artificial infection was left for 15 days for the development of disease symptom under an aerated poly bag. After 15 days symptoms was appeared on the leaf surface. The healthy and diseased leaves were detached separately. weight. was taken after proper washing and kept into oven at For diseased leaves: Wt. of diseased leaves - Wt. of oven dried leaves. Changes in Total Alkaloid: 20gm of powdered sample was soaked in 28% ammonium hydroxide solution and little Results and Discussion Changes in sugar: Carbohydrates or sugars are the chief photosynthetic products of the plants. The utilization of any particular sugar by fungi depends on its configuration as well as on the potential endowment of specific fungi. Fungi satisfy their carbon requirements from various carbohydrates present in host tissues by (i) Breaking the complex sugars to simpler utilizable form and by (ii) Enhancing the respiratory rate in diseased tissues. Di- and Polysaccharides are hydrolyzed by various hydrolyzing enzymes and are converted into hexose sugars which are preferably used by various pathogenic fungi mainly for two purposes i.e. to augment the required amount of energy for various metabolic process and for structural frame work of cell however, the rate of utilization varies with the variation of nature and type of fungi. Loss In Biomass: All living plant cells requires an abundance of water and an adequate amount of organic and inorganic nutrients in order to live and to carry out their physiological function. Plant absorbs water and inorganic (mineral) nutrients from the soil through their root system. The minerals and part of the water are utilized by the leaf and other cells for the synthesis of the various plant substances, but most of the water evaporates. On the other hand, nearly all organic nutrients of plants are produced in the leaf cells, following photosynthesis and are located downward and distributed to all the living plant cells. When a pathogen interferes with the upward movement of inorganic nutrients and water with the downward movement of organic substances, diseased conditions results in the parts of the plant denied these materials. The diseased parts in, turn, will be unable to carry out their own functions and deny the rest of the parts, their services or their products. For example if water movement to the leaves is inhibited the leaves cannot function properly, photosynthesis is reduced or stopped and few or no nutrients are available to move to the roots, which in turn become starved and diseased and may die or resulting in the loss of biomass Changes in Alkaloid Contents: The term Alkaloid has been proposed by pharmacists as basic nitrogen containing compounds from the plants and other natural resources in which at least one nitrogen atom forms a part of the cyclic system. The above stated definition is not fully satisfactory because a number of synthetic compounds satisfy all the above criteria of alkaloid except for the facts that they are not derived from biological resources. Thus in the modern chemistry they are defined as heterocyclic nitrogenous compounds. The further work carried out Codeine, Emetine, Morphine, Ergometrine, Serpentine and Quinine which are used to cure different ailments of human being. Previously it was reported [32] that Reserpine obtained from Rauvolfia serpentina have a strong hypotensive property, Vinblastine and Vincristin isolated from C. roseus as well as ergine, ergometrine, ergoline and isergine isolated from Argeria sp. are antidiabetic and hypoglyceamic Table 1: Changes in Sugar contents, Biomass and Alkaloids of C. roseus by C .gloeosporoides. 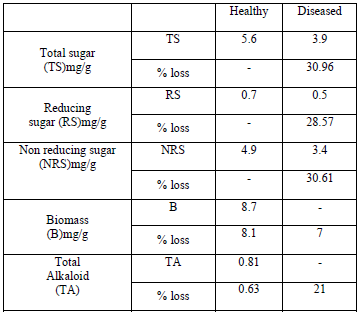
The data in table 1 clearly reveals that there was a general pattern of decrease of all kinds of sugars. 5.6mg/g total sugar, 0.7mg.g reducing sugar and 4.9mg/g Conclusions A general trend of decrease in sugar content of C. roseus was observed. Total sugar (TS) decreased by 30.36%, reducing sugar (RS) by 28.57% and non- reducing sugar by 30.61% respectively. The biomass was found to decrease up to 7% after the infection by C. gloeosporoides. The total alkaloid content in healthy leaves of C. roseus was 0.81 mg/g fresh leaves, however in diseased leaves it was decreased by 21%. Acknowledgements The authors are thankful to the Head, University Department of Botany, T.M. Bhagalpur University, Bhagalpur for providing laboratory facility and Ministry of Environment & Forests, Government of India for financial assistance. References 1.Vidhysekharan, P. and Kandaswamy, D. (1972). Carbohydrate metabolism of Phaseolus aurens infected with obligate and facultative parasites, Ind. Phytopath. 25(1): 2.Mirocha, C.J. and A.I. Zakai (1966). "Fluctuation in amount of starch in host plants invaded by rust and mildew fungi" phytopathology, 56: 3.Jayapal, R. and A. Mahadevan (1968). "Biochemical changes in Banana leaves in response to leaf spot pathogens" Ind. phytopath, 21: 4.Lily and Barnett (1951). Physiology of Fungi, McGraw Hill Book Co., Inc., New York, pp. 464. 5.Hasija, S.K. (1966). Additions to the fungi of Jabalpur, 28: 6.Panwar, K.S., Purohit, D.K. and Vyas, N.L. (1972). Two new species of Pestalotia from India. Curr. Sci. 41: 7.Srivastava, R.C. and Srivastava, G.C. (1976). A new leaf spot disease of Capparis horrida L. Curr. Sci. 45: 196. 8.Bhargava, S.N., R.S. Pandey, D.N. Shukla and D.W. Dwivedi (1981). Two new leaf spot diseases of medicinal plants. Nat. Acad. Sci. Letters. 4: 277. 9.Prasad, S.S. and Roy, A.B. Verma (1966). Occurrence of perfect stage of Colletotrichum gloeosporoides penz. on the leaves of Saraca indica L. Science & Culture, Vol. 32, pp. 10.Panwar, K.S., Purohit, D.K. and Vyas, N.L. (1972). Two new species of Pestalotia from India. Curr. Sci., 41: 11.Sah, R.P. (1984). A survey of medicinal plants of Santhal Pargana: Pathological and Biochemical studies of their antifertility properties. Ph.D. Thesis Bhagalpur University, Bhagalpur. 12.Das, N. (1985). Pathological and biochemical investigations of some medicinal plants used as expectorent Ph.D. Thesis, Bhagalpur University Bhagalpur. 13.Dutta, G.R. (1988). Pathological and pharmacological studies of some plants having hypoglycaemic properties, Ph.D. Thesis, Bhagalpur University, Bhagalpur. 14.Roy, A.K. and Chourasia, H.K.(1989). Aflatoxin problems in some medicinal plants under storage. Ind. J. Crude Drug Res. 26(3): 156. 15.Kumari, V. and Roy, A.K. (1990). Biodeterioration of Embelia ribes seeds under the influence of different relative humidities. Nat. Acad. Sci. letters. 13(5): 16.Sunita Kumari, M.M. Prasad and H.N.P. Singh (1993). Effect of physical factors on alkaloid contents of two antirheumatic seeds. Ind. Phytopath. Soc. Zonal chapter Meeting Bhagalpur, 20. 17.Dubois M, Gilles K.A., Hamilton, J.K., Rebers P.A., Smith F. (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 18.Peach, K. and M.V. Tracey (1955). "Modern methods of plant analysis" Springer Verlag, Berlin 11: 19.Mukherjee, B. (1953). Pharmaceutical codes, CSIR, New Delhi. 20.Kapoor, J.N. and Munjal, R.L. (1968). Additions to Indian fungi. Indian Phytopath, 21: 21.Singh, S.M. and Agarwal, G.P. (1973). On some folicolous Sphaeropsidales. Proc. Nat. Acad. Sci. India. 43B: 22.Prasad, K. Satya, Sailaja, K. Sri. (1995): Effect of VAM inoculation on Periwinkle (Catharanthus roseus CL.) G. Don. pp. 23.Groger, D. (1969). Anthranilic acid as precursor of alkaloids. Lloydia, 32: 24.Lucknar, M. (1969). In Biosynthese of der Alkaloide Monthes, K, Schutt, H.R.(eds) Berlin, Veb Verlay der Wiseen Schafer 25.Fielder E., H.P. Fielder, A. Gerhard, W. Keller, Schierlein, W.A., Konig, H. Zaher (1976). Staffwechsel produkte von 26.Verpoorte, R., Van der Heijden R., Moereno P.R.H. (1997). Biosynthesis of terpenoid indole alkaloides in Catharanthus roseus cells. In Cordell G.A.(Ed). The alkaloids (Academic Press, San Diego(CA) Vol. 49: 27. Mishra, P. and Kumar, S. (2000). Emergence of periwinkle catharanthus roseus as a model system for molecular biology at alkaloids: phytochemistry, pharmacology, plant biology and in vivo and in vitro cultivation, Journal of Medicinal and Aromatic Plant Sciences, 22: 28. Vander Herjden R., Jacobs D.I., Snoeijer W., Hallard, D., Verpoorte, R. (2004). The Catharanthus alkaloids, pharmacognosy and biotechnology. Current Medicinal Chemistry 11: 29. Shukla, A.K. (2005). Molecular studies on biosynthesis of shoot alkaloids in Catharanthus roseus (L) G. Don. Ph.D. thesis, Deptt. of Biochemistry Univ. of Lucknow, India. 30.Wagner H.S., Bladt and E.M. Zgain Ski (1984). Alkaloidal drugs. In Plant Drug Analysis, Springer Verlag, New York, 31. 32.Muller J.M., E. Schlitter and H.J. Bain (1952). Reserpine der sedative wirkstoffaas Rauwolfia serpentina Benth. Experientia 8: 388. 33.Hammounda, Y., M.M. Plat and J. Le Men (1963). Unnouvel alkaloide der Tecoma stans: La tecostanive. Annales de Pharmacie francaises 21: 34.Hammouda, Y., A. Rashid and M.S. Amer (1964). Hypoglycaemic properties of tecomine and tecostanine, Journal of Pharmacy and Pharmacology, 16: 35.Dhar, M.L., M.M. Dhar, B.N. Bhawan, B.N. Mehrotra and C. Roy (1968). Screening of Indian Plants for biological activity. Part I Ind. J. Exp. Bio., 6: 232. 36.Hammounda, Y. & N. Khallaufllah (1971). Stability of tecomine the major antidiabetic factor of Tecoma Stans Juss. Journal of Pharmaceutical Sciences, 60: Corresponding Author: |
|

