Adaptogenic and anti-stress activity of Withania somnifera in stress-induced miceAnju and Ashis Kumar Ghosh Department of Biochemistry Patna University, Patna, Bihar, India Abstract The aim of the study was to evaluate the effect of ethanolic extract of roots of Withania somnifera (23 mg/kg, p.o) on acute Key words: HPA system, Withania somnifera, Adrenocorticotropic hormone, Cortisol, Swim endurance test, Cold restraint stress. Introduction Adaptogens are naturally occurring substances found in plants. It is absolutely safe and Withania somnifera (Ashwagandha) has been used for thousands of years as a popular remedy for many conditions. Perhaps its main use, as described in Ayurvedic literature, is as a "rasayana" or rejuvenating drug. The word Ashwagandha indicates the equine (of horses) odour of the plant. Another name Avarada suggests the application of this plant for enhancing longevity. The root drug is considerd a tonic and roborant. The root of Withania somnifera is used to make the Ayurvedic sedative and diuretic "Ashwagandha", which is also considered an adaptogen. It is said to "protect the organism from illness" through maintaining the healthy balance of the physical energies [16, 31]. The root contains the steroid lactone withaferin A and related withanolides, besides various alkaloids [38, 39]. The sitoindosides IX and X represent Ashwagandha is one of the main herbs for promoting ojas and rejuvenating the body. It is well- known semen promoter and it treats impotency and infertility. It increases physical endurance and improves sexual function [17, Materials and Methods Plant material roots of Withania somnifera were collected, dried in shade, and finely powdered. The powder was soaked in absolute ethanol (95%) and left for 48 hours. The supernatant was collected and the residue was further soaked in absolute ethanol (95%) for 24 hours. The supernatant was collected and filtered. The filtrate was subjected to Rota vapour extraction at a temperature below 60oC for 24 hours. The concentrated form of the extract was obtained and The study was conducted on healthy, adult, male albino mice having a body weight of 35 + 5 g. They were acclimatized to laboratory condition for 2 weeks prior to experimentation. Animals were housed in propylene cages (6 mice/cage) in a mice experimentation laboratory at a temperature of 25oC + 2oC with 12 - 12 h dark -light cycle. They were provided with standard food and water ad libitum. Institutional animal ethical committee (I.A.E.C) approval was obtained before the experiment and care was taken to handle the mice in humane manner. All the chemicals used in the present study were obtained from Euro Diagnostics (Mumbai, India), India Scientific Company (Patna, Bihar) and Bihar Scientific Corporation (Patna, Bihar). Experimental Design The adult animals (8 weeks old) were divided into 4 groups (n = 6 in each group) as follows: Group I consisted of Normal control (NC), these mice remained undisturbed in the home cage throughout the experimental period. Group II consisted of Stress control (SC), which were fed with equivolume of distilled water orally for 7 days. Group III (Stress+P.ginseng) consisted the standard group, these mice were fed with aqueous root powder of Panax ginseng, (p.o) for 7 days. Group IV consisted of (Stress+W.somnifera), treatment group which were fed with ethanolic extract of Withania somnifera, (p.o) for 7 days. Stress Procedure Swim Endurance Test: The mice in group IV were given ethanolic extract of Withania somnifera, 23 mg/kg, (p.o), using oral gauge for 7 days. The standard group (III) was administered water soluble root powder of Panax ginseng 100 mg/kg, (p.o), while the stress control group (II) was administered distilled water for 7 days orally. On the 8th day, the animals were allowed to swim till exhausted in a propylene tank of dimension 24 cm* 17 cm* 14 cm, filled with water to a height of 10 cm. The end point was taken when the animals drowned and 'swimming time' for each animal was noted. The mean swimming time for each group was calculated and the data was statistically analyzed. Cold Restraint Stress: The mice in group IV were given ethanolic extract of Withania somnifera 23 mg/kg, (p.o), using oral gauge for 7 days. The standard group (III) was administered water soluble root powder of Panax ginseng 100 mg/kg, (p.o), while the stress control group (II) was administered distilled water for 7 days orally. On the 8th day, the animals were individually placed in plastic containers of capacity 350 ml. They were immobilized in their normal position, using adhesive tape. The containers were placed in a cold chamber maintained at 4oC for 1 hour. The blood was collected by orbital sinus veinpuncture method in a heparinised tube and the following investigations were carried out. Total WBC count was done using Neubauer's chamber, blood glucose was determined by GOD/POD method, plasma cortisol was determined by Enzyme Linked Immunosorbent Assay (ELISA) [32], serum triglyceride was determined by Statistical Analysis Data was analyzed by application of one way analysis of variance (ANOVA) using Graph pad in stat software. P<0.01 was considered to be significant. Results and Discussion Acute toxicity studies with the extract revealed that LD50 is 1750 mg/kg body weight, (p.o). As shown in figure 1, the extract of Withania somnifera improves swim duration in mice. Mice pretreated with ethanolic extract of Withania somnifera 23 mg/kg, (p.o), and water soluble root powder of Panax ginseng 100mg/kg, (p.o), show significant improvement in the swimming time (P<0.01), as compared to control. (n = 6 in all groups, SC vs S+W.somnifera, P<0.01; SC vs S+P.ginseng, P<0.01; One way ANOVA, P<0.01, F = 41.336; Fig. 1). The induction of cold restraint stress led to a rise in total WBC count, blood glucose, plasma cortisol and serum triglyceride levels. All the two treatments produced a significant reduction in total WBC count (P<0.01), as compared to controls. (n = 6 in all groups, NC vs SC, P<0.01; SC vs S+W.somnifera, P<0.01; SC vs S+P.ginseng, P<0.01; One way ANOVA, P<0.01, F = 6.006; Fig. 2). The blood glucose was significantly increased, when the animals were subjected to cold restraint stress compared to control (P<0.01). Pretreatment of animals with the extract of Withania somnifera 23 mg/kg, (p.o), or water soluble root powder of Panax ginseng 100 mg/kg, (p.o), prevented this (P<0.01). (n = 6 in all groups, NC vs SC, P<0.01; SC vs S+W.somnifera, P<0.01; SC vs S+P.ginseng, P<0.01; One way ANOVA, P<0.01, F = 60.373; Fig. 3). The plasma cortisol level which was found to be elevated in the animals subjected to cold restraint stress was significantly reduced by all the four treatments (P<0.01), compared to controls. (n = 6 in all groups, NC vs SC, P<0.01; SC vs S+W.somnifera, P<0.01; SC vs S+P.ginseng, P<0.01; One way ANOVA, P<0.01, F = 92.616; Fig. 4). The triglyceride level was increased in the animals subjected to cold restraint stress compared to control (P<0.01). However, no significant change in the serum cholesterol level was observed. Treatment of animals with the extract of Withania somnifera 23 mg/kg, (p.o), or water soluble root powder of Panax ginseng 100 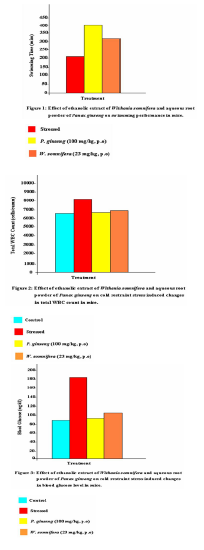
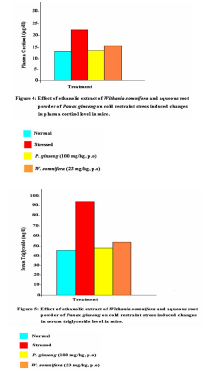
mg/kg, (p.o), before subjecting them to cold restraint stress, prevented the increase in serum triglyceride levels (P<0.01). (n = 6 in all groups, NC vs SC, P<0.01; SC vs S+W.somnifera, P<0.01; SC vs S+P.ginseng, P<0.01; One way ANOVA, P<0.01, F = 98.553; Fig. 5). The testing of the physical endurance of mice, after Thus, on the basis of the above findings it is concluded that the extract of Withania somnifera improves the swim duration in mice and prevented the increase in total WBC count, blood glucose, plasma cortisol, and serum triglyceride levels. Acknowledgements This study was supported by the Department of Biochemistry, Patna University, Patna.
Nutritional and Botanical
References 1.Lazarev, N.V. 7th All -union Cong. Physiol., Biochem., Pharmacol., p. 579. Medgiz , Moscow 1947. 2.Selye, H. Endocrinology 1937; 21/2:169. 3.Selye, H. Nature 1938; 141:926. 4.Brekhman, 1. Man and Biologically Active Substances. 5.Selye, H. Nature 1936, 138:32. 6.Selye, H. Am. J. Physiol. 1938,123:758. 7.Bhattacharya, S.K., Goel, R.K. Kaur, R., and Ghosal, S. Phytotherapy Res. 1987;1/1:32. 8.Ghosal, S., Bhattacharya, et al., Phytotherapy Res. 1989:3/5:201. 9.Godhwani, S., Godhwani, J.L., and Vyas, D.S. J. Ethnopharmacol. 1987; 21:153. 10.AB Negrao, PA Deuster, PW Gold, A Singher and GP Chrousos, Individual reactivity and physiology of the stress response, 54 (3) (2000), 11.Ghosal S, et al. Immunomodulatory and CNS effects of sitoindosides IX and X, two new glycowithanolides from Withania somnifera. Phytother Res 1989; 3:201. 12.Agarwal R, et al. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J. Ethnopharmacol. 1999; 67:27. 13.A Panossian., G Wikman and H Wagner. Plant adaptogens, III, Earlier and more recent aspects and concepts on their mode of action, Phytomedicine, 6 (4), (1999), 14.Archana R, et al. 15.Bhattacharya S, et al. 16.Bhattacharya, S.K. and Muruganandam, A.V., 2003. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacology, Biochemistry and Behaviour, 75, 17.Bhattacharya, S.K., Goel, R.K. Kaur, R., and Ghosal, S. Phytotherapy Res. 1987; 1/1:32. 18.Biswas NM, Sengupta R, Roychaudhuri G, Chattopadhyay A, Sarkar M. Prevention of adrenocortical hyperactivity by dietary casein in rats exposed to forced swimming stress. Indian J. Exp. Biol 2001; 19.Bone K. Clinical Applications of Ayurvedic and Chinese Herbs: Monographs for the Western Herbal Practitioner. Warwick, Queensland: Phytotherapy Press; 1996. 20.Bove, Mary, ND. Adrenal Function, Stress and Botanical Medicine. Medicines from the Earth Proceedings. Black Mountain, NC:2003. 21.Brekhman, I.I., and Dardymov, I.V. Ann Rev. Pharmacol. 1969; 9:410. 22.Brekhman, I.I. and Dardymov, I.V., 1969. New substances of plant origin which increase non- specific resistance.Annual Review of Pharmacology, 9, 23.Carlini, E.A., 2003. Plants and the central nervous system. Pharmacology, Biochemistry and Behaviour, 75, 24.Carrasco, Gonzalo A. and Van de Kar, Louis D., 2003. Neuroendocrine Pharmacology of stress. European Journal of Pharmacology, 463, 25.Chrousos, G.P and Gold, P.W., 1992. The concepts of stress and stress system disorders. Journal of the American Medical Association, 267, 26.Chrousos, George P., 1998, Stressors, stress, and neuroendocrine integration of the adaptive response. In: Peter Csermely (Ed.), Stress of Life: From Molecules to Man. Annals of the New York Academy of Sciences. The New York Academy of Sciences, New York. 27.Cole TG, Klotzsch SG, Mc Namara J. Measurement of triglyceride concentration. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of Lipoprotein testing. Washington: AACC Press; 1997. 28.Dhuley J. Adaptogenic and Cardioprotective action of ashwagandha in rats and frogs. J. Ethnopharmacol 2000; 70:57. 29.Frazer, A.C. 1961. Role of lipids in normal metabolism. Fed. Proc. 20 (No. 1, Part 3, Suppl. 30.George P, Chrousos MD, Philip W, Gold MD. The concept of stress system disorders. J Am Med Assn 1992; 31.Ghosal, S., Jaiswal, D.K, Singh, S.K, and Srivastava, R.S. : Phytochemistry 1985; 24/4:831. 32.Glick D, Vonredlich D, Levine S. Fluorometric determination of Corticosterone and Cortisol in 33.G Sundaresan, N Suthanthirarajan and A Namasivayam, Certain immunological parameters in subacute cold stress, Ind. J. Physiol. Pharmacol., 34 (1) (1990), 34.Gupta S, Aslakson E, Gurbaxani BM, et al. Inclusion of the glucocorticoid receptor in a hypothalamic pituitary adrenal axis model reveals bistability. Theor Biol Med Model 2007 Feb 14; 4:8. 35.Habib, Kamal E., Gold, Philip W. and Chrousos, George P., 2001. Neuroendocrinology of stress. Neuroendocrinology, 30(3), 36.Hoffman, David, FNIMH, AHG. Medical Herbalism. The Science and Practice of Herbal Medicine. Healing Arts Press, 2003. 37.Howe, Gregg A. and Schilmiller, Anthony L., 2002. Oxylipin metabolism in response to stress. Current opinion in Plant Biology, 5, 38.Hunter I, et al. Separation of withanolides by high- pressure liquid chromatography with coiled columns. J. Chromatogr 1979; 170:437. 39.H Varley, AH Gowenlock and M. Bell, In: Practical Biochemistry, 5th edition, William Heine Mann Medical Books Ltd, London, (1984), 40.Indian Materia Medica by Dr. K.M Nadakarni, Vol. 2, Popular Prakashan, Reprint 2000. 41.Indian Pharmacopoeia. 42.Izawa S, Sugaya N, Ogawa N, et al. Episodic stress associated with writing a graduation thesis and free cortisol secretion after awakening. Int J Psycophysiol. 2007 May; 43.Juvekar AR, Nachankar RS. Restraint stress- induced changes and their modification by Spirulina platensis in albino rats: An experimental study. Acta Hort (ISHS) 2005; 44.Kandil F, et al. Flavonol glycosides and Phenolics from Withania somnifera. Phytochem 1994; 37:1215. 45.Kelly, Gregory S, ND. "Nutritional and Botanical Interventions to Assist with the Adaptation to Stress." Alternative Medicine Review; Vol. 4 46.Kirson I, et al. Constituents of Withania somnifera Dun. Part XII. The withanolides of an Indian Chemotype. J Chem Soc (C) 1971; 2032. 47.Kirtikar, KR.; Basu, BD. In: Kirtikar KR, Basu BD., editor. Indian Medicinal Plants. II. II. Allahabad, India, Lalit Mohan Basu Publications; 1935. 48.Klauer, Kevin DO, Adrenal Insufficiency and Adrenal Crisis., www.emedicine.com Dec.7, 2004. 49.Klein, R., Kindscher, K., Botanical Medicines with Stress Adaptogen Properties in Ethnobotanical Literature: A 50.Lavie D, et al. Constituents of Withania somnifera Dun. Part IV. The structures of withaferin A. J Chem Soc 1965;7517. 51.Lavie D, et al. Constituents of Withania somnifera X.The structure of withanolide D. Isr J Chem 1968; 6:671. 52.Mc Ewen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev 2007; 53.Mc Ewen, Bruce S., 1999. Stress and the aging hippocampus. Frontiers in Neuroendocrinology, 20, 54.Mc Ewen, Bruce S., 2002, The End to Stress As We Know It. Joseph Henry Press, Washington. 55.Mc Gowan MW, Artiss JD, Strandbergh DR, Zak B.A 56.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): A review, Altern Med Rev 2000; 57.Munck A, 58.Panossian, A., Adaptogens, Tonic Herbs for Fatigue and Stress, Alternative and Complementary Therapies, Corresponding Author: Email: danju951@gmail.com; anju.fr@gmail.com |
|

